A gas detector is a special gas safety concentration detection instrument, equipped with various types of gas sensors, such as electrochemical sensors, infrared sensors, semiconductor sensors and so on. Its working principle is to detect the gas concentration in the environment through the sensor, and the detection result is converted into an electrical signal, and then processed and analyzed, and finally shows the gas concentration or alarm signal. The core of the gas detector is the sensor, which according to the different detection gases, the working principle of the distinction is not the same. Common working principles of gas detectors and monitors include catalytic combustion, electrochemical, infrared, PID, semiconductor and other principles.
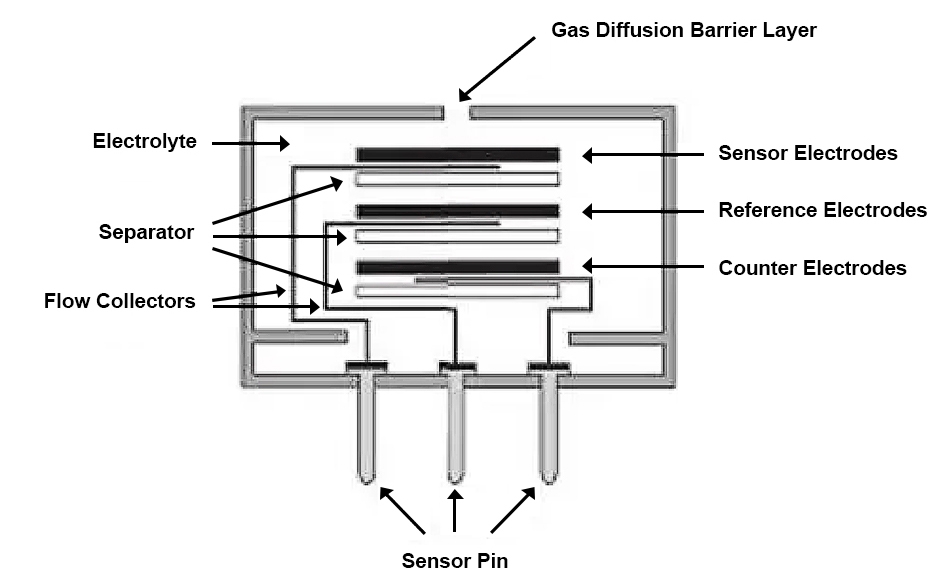
Electrochemical Working Principle
Electrochemical gas detectors work by means of a sensor that reacts with the measured gas and generates an electrical signal proportional to the gas concentration. The gas being measured first reacts with the sensor through tiny capillary-type openings, then a water-repellent barrier, and finally reaches the electrode surface. A moderate amount of gas is next allowed to react with the sensing electrode to form an adequate electrical signal while preventing electrolyte leakage from the sensor. The gas diffusing through the barrier reacts with the sensing electrode, which can be either an oxidizing or reducing mechanism.
These reactions are catalyzed by electrode materials designed for the gas being measured, and a current proportional to the concentration of the gas being measured flows between the positive and negative electrodes through a resistor connected between the electrodes. The current is measured to determine the gas concentration. Electrochemical gas detectors are widely used in the laboratory field because of their low power consumption, good linearity and repeatability, and high sensitivity.
Semiconductor Working Principle
Semiconductor sensors use semiconductor material and when the target gas comes in contact with the semiconductor, the conductivity changes. This change can be used to detect the presence and concentration of the gas. It is highly influenced by the environment in which it is measured and therefore the output linearity is unstable. Due to the very sensitive response, semiconductor sensors are now widely used in the field of measuring the microleakage phenomena of gases.
Catalytic Combustion Working Principle
Catalytic sensors are mainly used to detect combustible gases. It is the use of Wheatstone bridge principle, by the detection element and compensation element paired composition of the bridge arm, flameless combustion occurs on the surface of the sensitive body of the detection element when encountering flammable gases, the sensitive body temperature rises, the resistance of the temperature-sensitive material increases, the bridge output voltage becomes larger, the voltage change with the increase in the concentration of the gas and the increase is proportional to the increase in the amount of the change, according to the determination of the size of the change in the output signal of the bridge can be determined by the detection of the gas. According to the size of the change of the output signal of the bridge, the concentration of the detected gas can be determined.
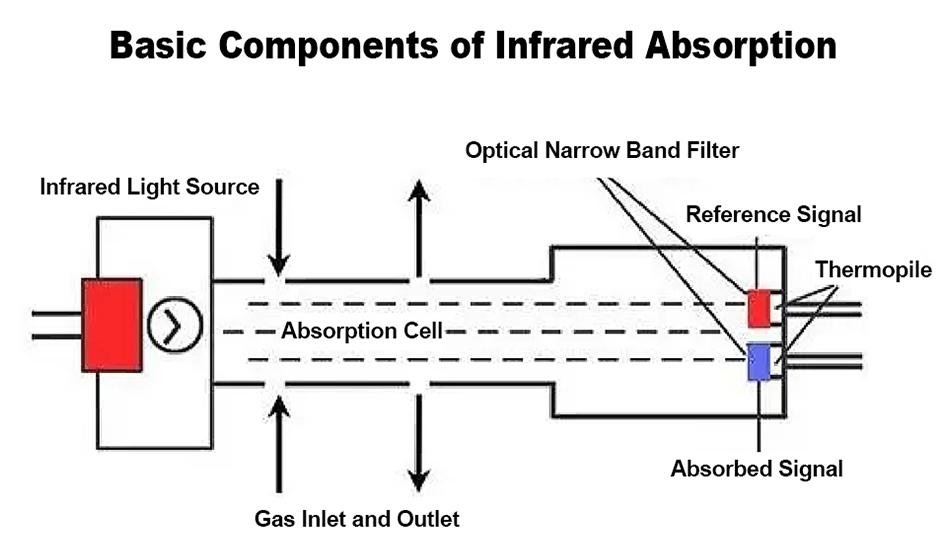
Infrared Working Principle
Infrared gas detector principle is to be measured continuously through a certain length and volume of gas containers, from the container can be translucent two end faces in an end face side into a beam of infrared light, and then in the other end face to determine the intensity of infrared radiation, and finally based on infrared absorption and light-absorbing material concentration is proportional to the size of the gas can be known to the concentration of the gas being measured.
Infrared gas detector has high sensitivity, ppm level gas concentration can be distinguished even if there is a small change. It has a wide measuring range: the upper limit of the analyzed gas is up to 100% VOL, and after refinement, it can also measure the analysis of ppb level gases. The infrared gas detector is mainly suitable for detecting Methane (CH4), Carbon Dioxide (CO2), Nitrogen Dioxide (NO2), Carbon Monoxide (CO), Sulfur dioxide (SO2), Ammonia (NH3) and other gases. It can also detect most organics (HC), organic volatile compounds (VOC) and other gases.
Photoelectric Ionization (PID) Working Principle
PID gas sensors work by means of a source of ultraviolet light, in which chemical substances are excited to produce positive and negative ions that can be detected by the detector. Molecules in the absorption of high-energy ultraviolet light will produce ionization, in this excitation molecules produce negative electrons and constitute positive ions. The current generated by the ionized particles is amplified by the detector, and the concentration in ppm can be shown on the exterior.
